| Ch 1. Basics | Multimedia Engineering Fluids | ||||||
|
Mass Density |
Ideal Gas Law |
Viscosity |
Surface Tension |
Vapor Pressure |
|||
| Vapor Pressure | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Fluid Statics |
| 3. Kinematics |
| 4. Laws (Integral) |
| 5. Laws (Diff.) |
| 6. Modeling/Similitude |
| 7. Inviscid |
| 8. Viscous |
| 9. External Flow |
| 10. Open-Channel |
| Appendix |
| Basic Math |
| Units |
| Basic Fluid Eqs |
| Water/Air Tables |
| Sections |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| ©Kurt Gramoll |
|
|
||
|
|
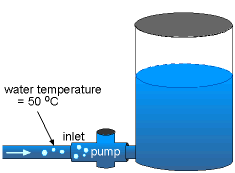
Cavitation occurs when the absolute pressure is less than or equal to the vapor pressure (pv), so that the water is allowed to "boil". For water temperature of 50 oC, the vapor pressure is 12.33 kPa. Since the operating pressure of the pump on the inlet side is 6 kPa, which is less than the pv, cavitation will occur. On the other hand, for water temperature of 20 oC, the vapor pressure is 2.338 kPa. The operating pressure of the pump on the inlet side is now greater than the pv, hence cavitation will not occur. |
|