| Ch 4. Energy Analysis | Multimedia Engineering Thermodynamics | ||||||
|
Steady-flow Process |
Steady-flow Devices (1) |
Steady-flow Devices (2) |
Steady-flow Devices (3) |
Unsteady-flow Process |
|||
| Steady-flow Devices (1) | Case Intro | Theory | Case Solution |
| Chapter |
| 1. Basics |
| 2. Pure Substances |
| 3. First Law |
| 4. Energy Analysis |
| 5. Second Law |
| 6. Entropy |
| 7. Exergy Analysis |
| 8. Gas Power Cyc |
| 9. Brayton Cycle |
| 10. Rankine Cycle |
| Appendix |
| Basic Math |
| Units |
| Thermo Tables |
| Search |
| eBooks |
| Dynamics |
| Fluids |
| Math |
| Mechanics |
| Statics |
| Thermodynamics |
| Author(s): |
| Meirong Huang |
| Kurt Gramoll |
| ©Kurt Gramoll |
|
|
||
|
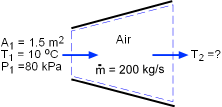
Air temperature at the exit of the wind tunnel needs to be determined to see if it is safe to exhaust the air to ambient. Assumptions:
|
||
|
|
(1) Determine the velocity at the inlet of the diffuser To determine the velocity at the inlet, the specific volume of the air needs to be first determined. Since the air in the tunnel is an ideal gas, it obeys the ideal-gas equation of state. Pv = RT where The specific volume can be determined at the inlet conditions: v1 = RT1/P1 = 287(273+10)/80,000 = 1.015 m3/kg The velocity can be calculated using the following equation: v1 = (2) Determine the temperature at the exit of the diffuser Under the stated assumptions and observations, the energy balance for the steady-flow through the diffuser can be expressed as (h2 - h1) + ( v22 - v12)/2 = 0 h2 = h1 - ( v22 - v12)/2 The enthalpy of air at the diffuser inlet can be determined from the air table to be h1 = h@283 k = 283.14 kJ/kg Assume the velocity at the exit is 0, then the enthalpy reaches the maximum value. h2 = 283.14 - ( 02 - 135.32)/2/1,000 = 292.3 kJ/kg From the air table, the temperature corresponding to this enthalpy value is T2 = 292 K = 19oC < 35 oC which shows that the air is safe to exhaust to the outside environment. |
|